- Home >> News >> Research Progress
Research Progress
Cell-cell adhesion facilitates receptor aggregation and raft phase separation
Biological cells use membrane-anchored receptor proteins to physically interact and, thereby, identify and communicate with other cells. Many of these receptor proteins have been reported to associate with nanoscale molecular clusters enriched in sphingolipid and cholesterol. These nanoscale clusters – which are also called ‘lipid rafts’ – can be stabilized and driven to coalesce into platforms that function in membrane signaling, which is a subject of a long-standing debate and intense research. A central question is how the lipid rafts respond to physical contacts between cells.
Researchers from the Institute of Mechanics, Chinese Academy of Sciences and the Nanjing University, and the Institute of Physics, Polish Academy of Sciences, discuss the effect of cell-cell adhesion on the stabilization and coalescence of lipid rafts. They propose a statistical-mechanical model for a membrane system in which the membrane-anchored receptors and ligands associate weakly with lipid rafts. Their results show that intercellular receptor-ligand binding and membrane shape fluctuations can lead to aggregation of the raft-associated receptors even if raft domains are thermodynamically unstable in free, non-adhering membranes. They discover that thermally excited undulations of the membranes can either facilitate or impede the adhesion-induced receptor aggregation, depending on the strength of the receptor-ligand binding, which has deep implications for our understanding of physical mechanisms of cellular signaling. In fact, the strength of the receptor-ligand binding can be actively modulated in living cells. For example, the interactions between the T-cell receptor (TCR) and the major histocompatibility complex (MHC) are modulated by peptides displayed at the MHC molecules, and the ability of the TCR to discriminate ‘foreign’ from ‘self’ peptides is a requirement of an effective adaptive immune response. Since the MHC molecules are present in lipid rafts, their theory predicts – in this particular case – how the peptide-modulated strength of the TCR-MHC binding can regulate the TCR aggregation. Their theory, however, is rather general and applicable to various types of receptors that function in cellular signaling, immunological responses or other biological processes.
This work entitled “Intercellular Receptor?Ligand Binding and Thermal Fluctuations
Facilitate Receptor Aggregation in Adhering Membranes” has been published in Nano Letters (2020, 20: 722-728).
The paper link: https://pubs.acs.org/doi/10.1021/acs.nanolett.9b04596
The authors acknowledge support from the Programs in the National Key Research and Development Program of China (2016YFA0501601), the National Natural Science Foundation of China (21504038, 21973040, 11902327, and 11972041), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB22040102), and the Opening Fund of State Key Laboratory of Nonlinear Mechanics.
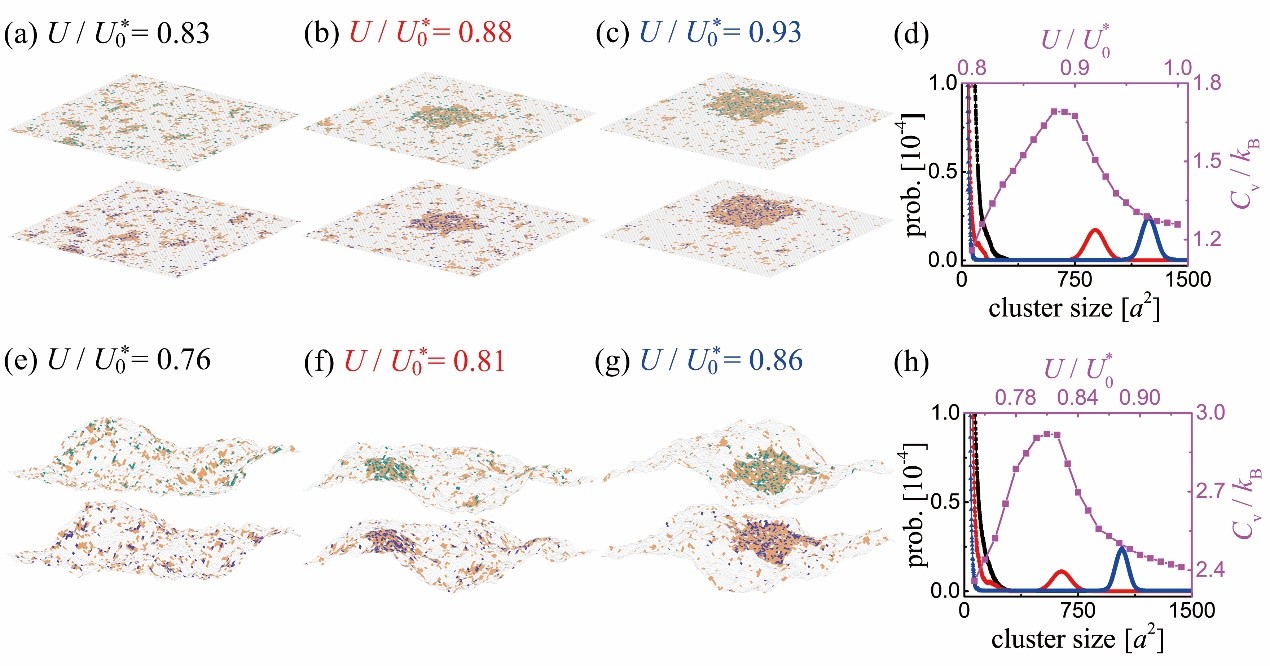
Figure 1. Snapshots from MC simulations of planar (a-c) and fluctuating (e-g) membranes. Lipid rafts are shown in orange; membrane-anchored receptors, in green; and membrane-anchored ligands, in blue. The values of the contact energy U are indicated. (d) Size distributions of raft clusters for U/U0* = 0.83 (black), 0.88 (red), and 0.93 (blue) as well as Cv as a function of U/U0* (purple) obtained from the MC simulations of the planar membranes. (h) Size distributions of raft clusters for U/U0* = 0.76 (black), 0.81 (red), and 0.86 (blue) as well as the Cv versus U/U0* curve (purple) obtained from the MC simulations of the fluctuating membranes.
