In the catalytic combustion of carbon-based fuels, the relationship of qualitative and quantitative analysis among the adsorption of reactants, intermediate species and the active center of catalysts presents a long-standing scientific problem and challenge. The CO has been widely accepted as a carbon-based fuel with the simplest probe molecule and molecular structure, and thus its catalytic combustion reaction has the dual value of basic and application research. Both catalytic combustion (CC) and chemical looping combustion (CLC) are promising technologies for energy saving and emission reduction of CO2 in treatment of steelmaking off-gas (CO), which is beneficial to achieving the goal of "carbon peak and carbon neutral". Therefore, it’s necessary to bridge the missing gap between the qualitative and quantitative description in understanding the evolution behaviors and fundamental quantitative reaction mechanism over the model catalyst for CC and CLC reaction.
In view of the energy waste and environmental pollution caused by direct combustion of converter steelmaking off-gas, the researchers from institute of Mechanics, Chinese Academy of Sciences, Tianjin University of Science and Technology and Aalto University cooperated to make research progress on the microscopic reaction mechanism of CO in CC and CLC processes over the cubic Cu2O catalyst.
The present investigation reports and compares the evolution behavior and quantitative reaction mechanisms of cube Cu2O model catalyst for CC and CLC reactions. The Cu2O-CC exhibited the higher activity and stability than Cu2O-CLC. The typical characterization results suggested that the only surface unstable Cu2O was oxidized to CuO, and the excellent synergistic effect of metal-oxide interface (1 0 0) between Cu+/Cu2+ and active lattice oxygen species for Cu2O-CC reaction. But CLC reaction led Cu2O structure collapse which caused low stability and agglomeration of CuOx species (Fig.1). Then three different active oxygen species (surface cycle lattice oxygen, bulk lattice oxygen, and adsorbed oxygen) and the detailed reaction pathways were proposed by the in situ IR spectroscopy, isotopic (18O2) transient exchange experiments and DFT simulation (Fig.2 and 3). The intrinsic activity of surface cycle lattice oxygen was higher in terms of TOF (13.5×10-3 s-1) and facile formation of C16O18O on the cubic interface of Cu2O-CC through adsorbed CO during CC process. The contribution degrees of Mars-van-Krevelen and Langmuir–Hinshelwood mechanisms for CC and CLC reactions were 76.6% and 23.4% for CC over Cu2O, and 89.7% and 10.3% for CLC on Cu2O, respectively. These fundamental results are beneficial to making a comprehensive understanding of the actual surface reaction process on cubic Cu2O catalyst in the CC and CLC, and also provide theoretical support to the advanced catalyst design and intrinsic mechanism research for CC and CLC processes.
The related results were published in Applied Catalysis B: Environmental (https://doi.org/10.1016/j.apcatb.2022.121296) on March 8th 2022. The first author of this paper is Kang Running, PhD student of the Institute of Mechanics, and Bin Feng, associate researcher, is the first corresponding author. This work was supported by the National Natural Science Foundation of China (52176141) and the China Scholarship Council (CSC).
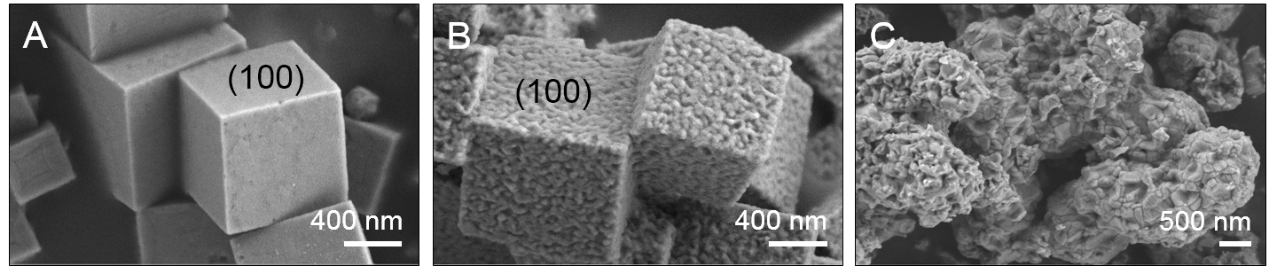
Fig. 1. SEM images of the fresh Cu2O (A) and used catalyst for CC (B) and CLC (C) processes
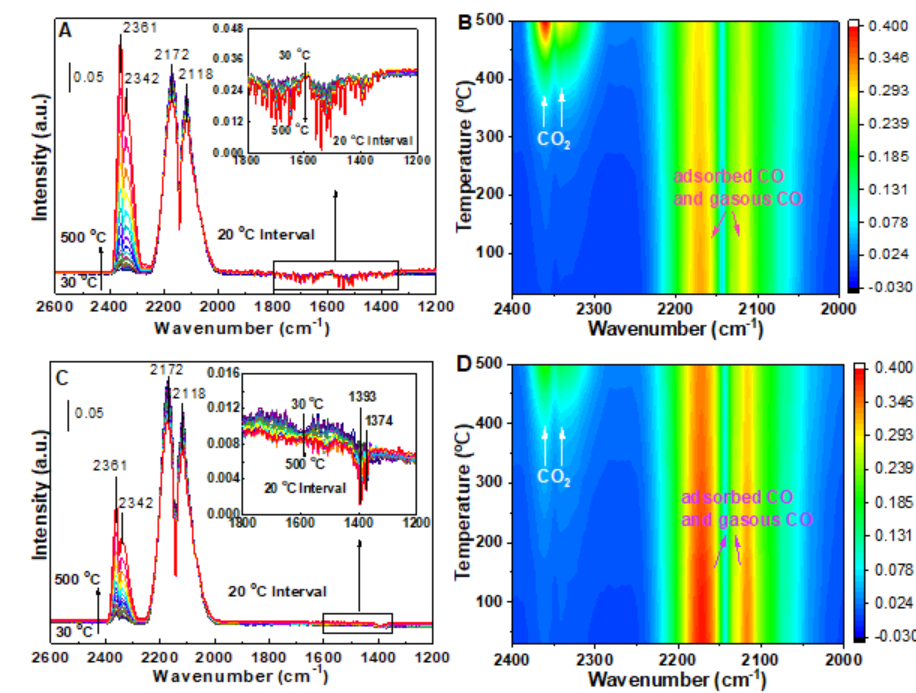
Fig.2. In situ IR spectra of fresh Cu2O and corresponding mapping results under (A, B) 10 vol.% CO+21 vol.% O2/Ar, and (C, D) 10 vol.% CO/Ar continuous stream.
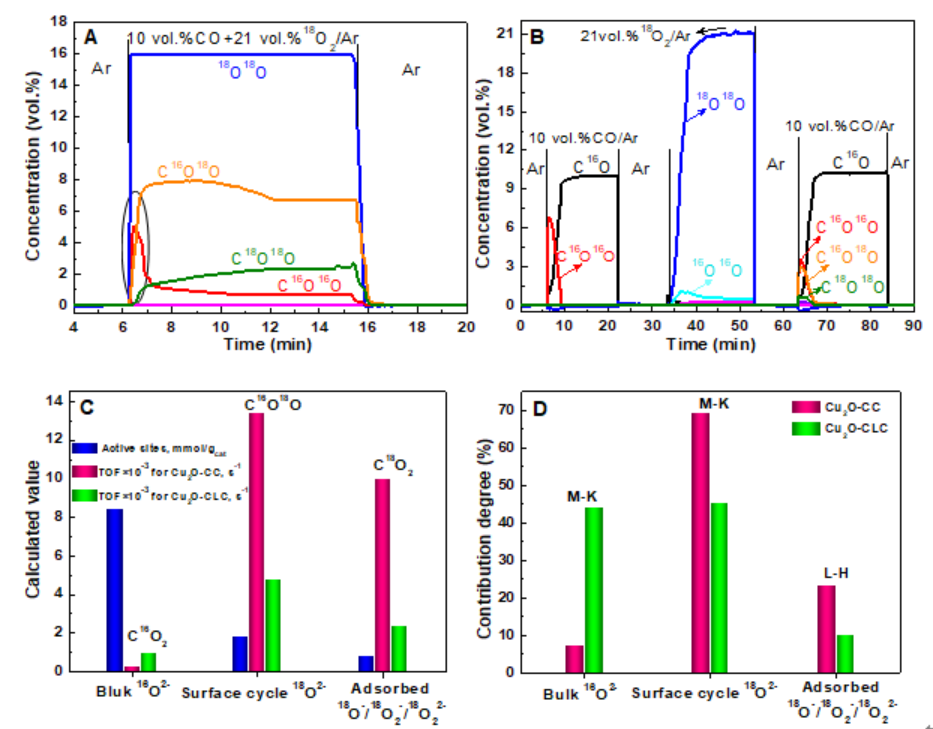
Fig. 3. Isotopic (18O2) exchange experiments over Cu2O for CC (A) and CLC (B) processes at 300 oC; the amount of different active sites and TOF (C), and contribution degree of different mechanisms on active oxygen species (D) for Cu2O-CC and Cu2O-CLC
Contact
MS. zhang
Institute of Mechanics, Chinese Aademy of Siences
Tel : +86-10-82544075
Email: zhanglingfang@imech.ac.cn
Web: http://english.imech.cas.cn/



